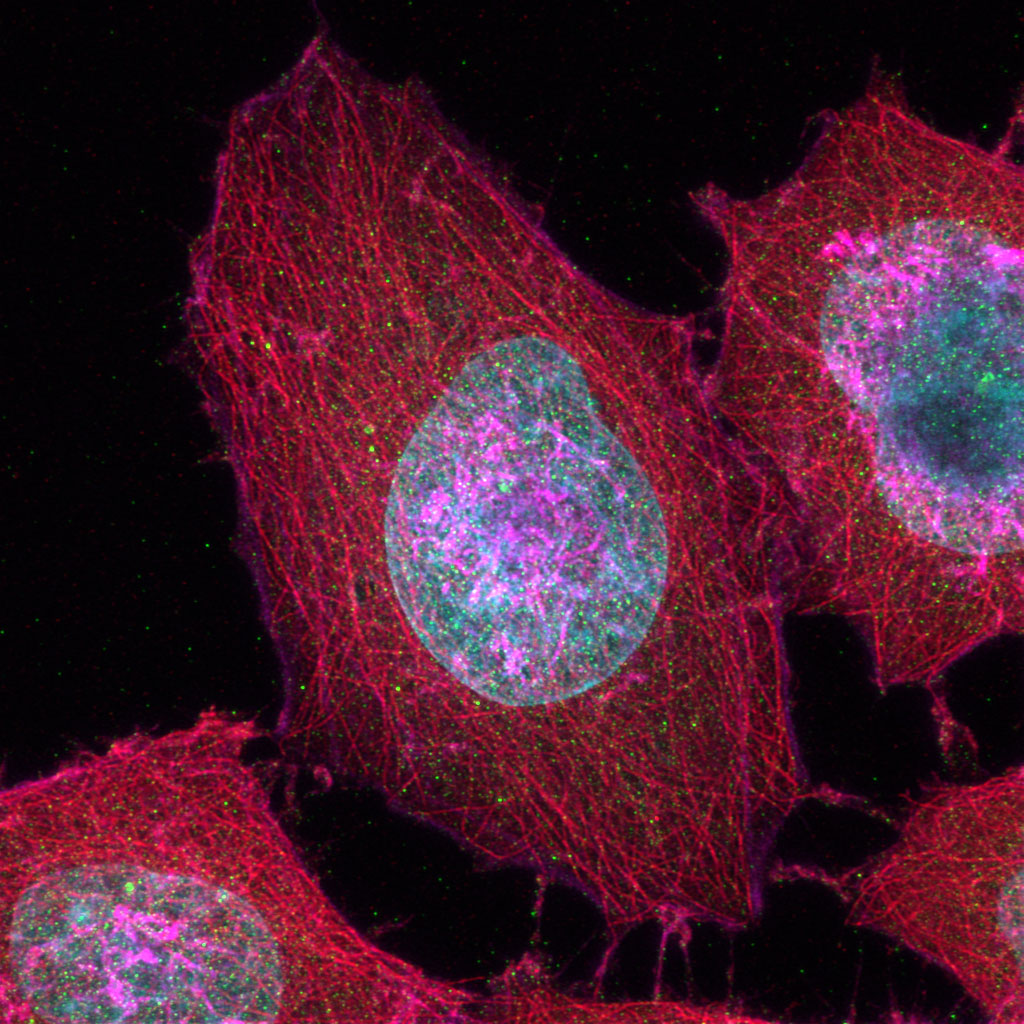
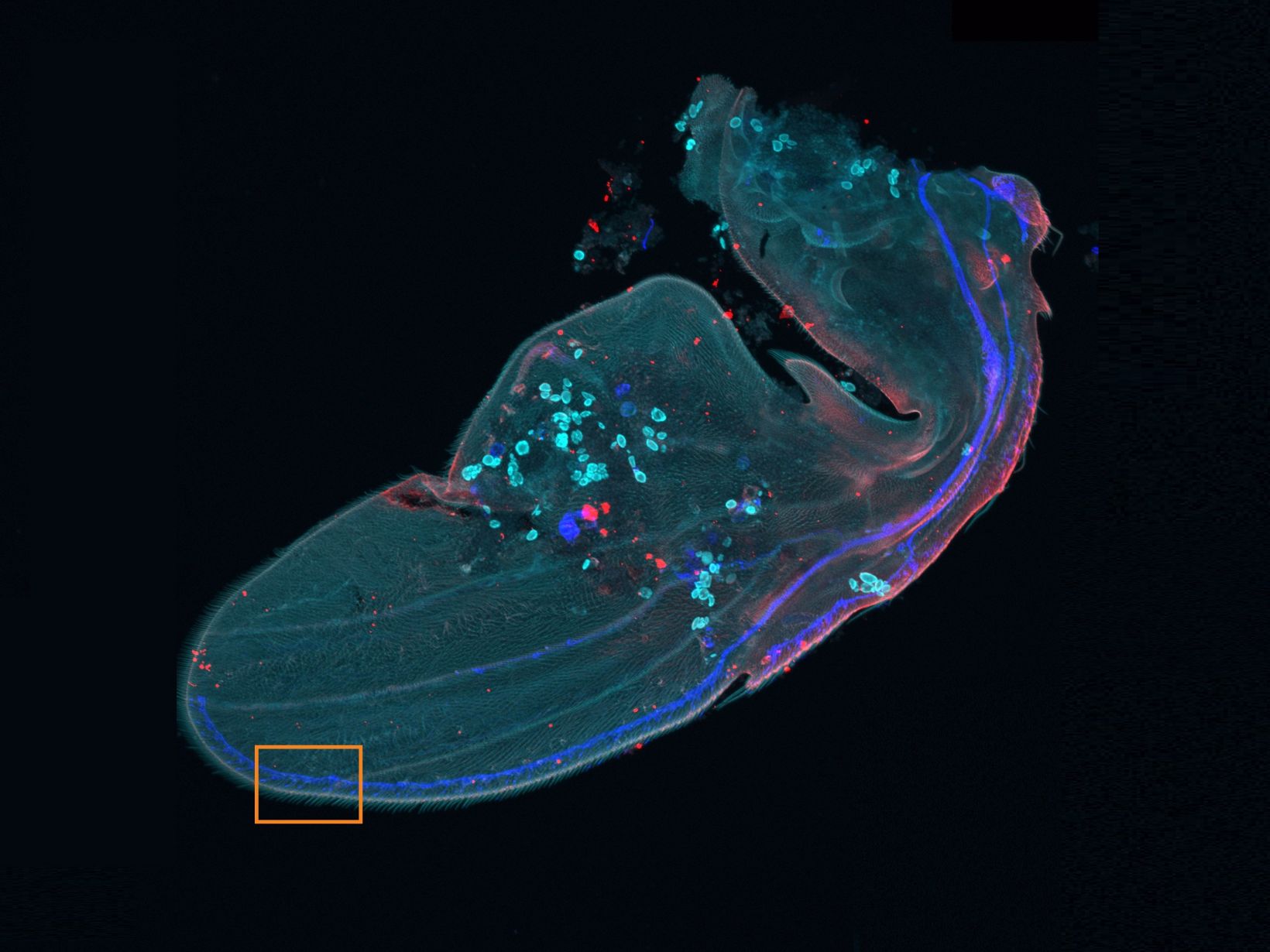
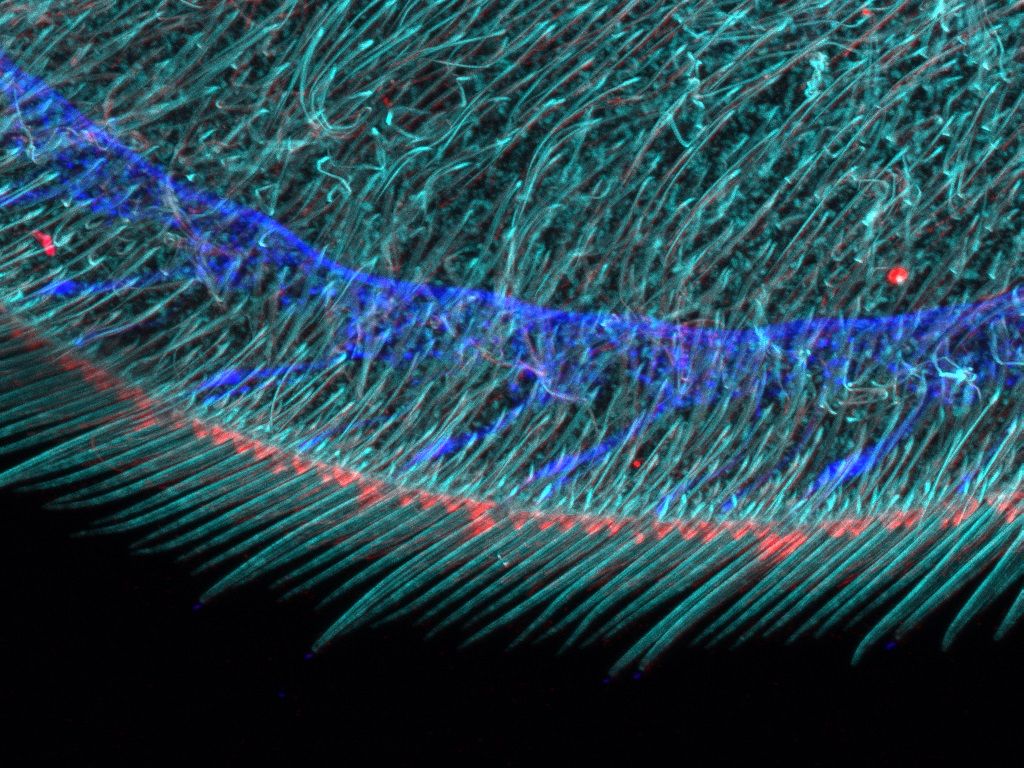
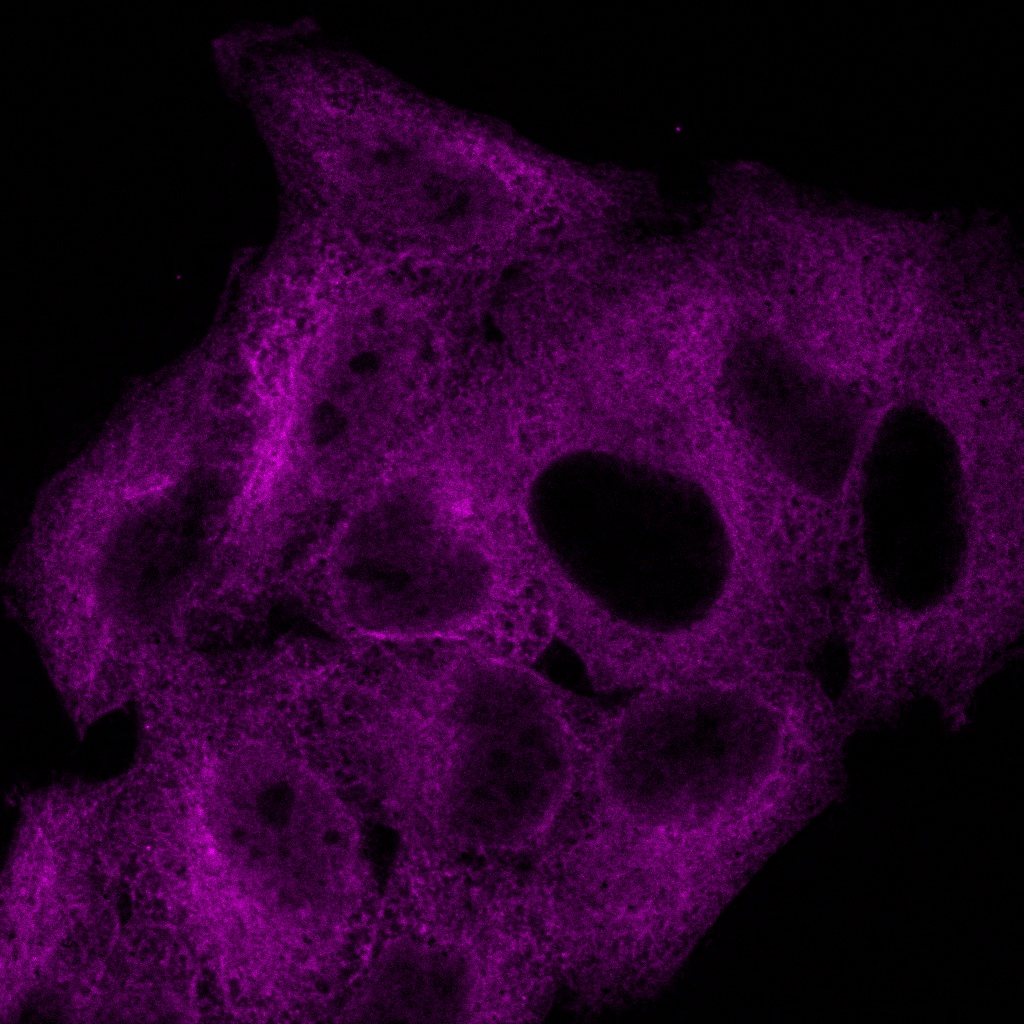
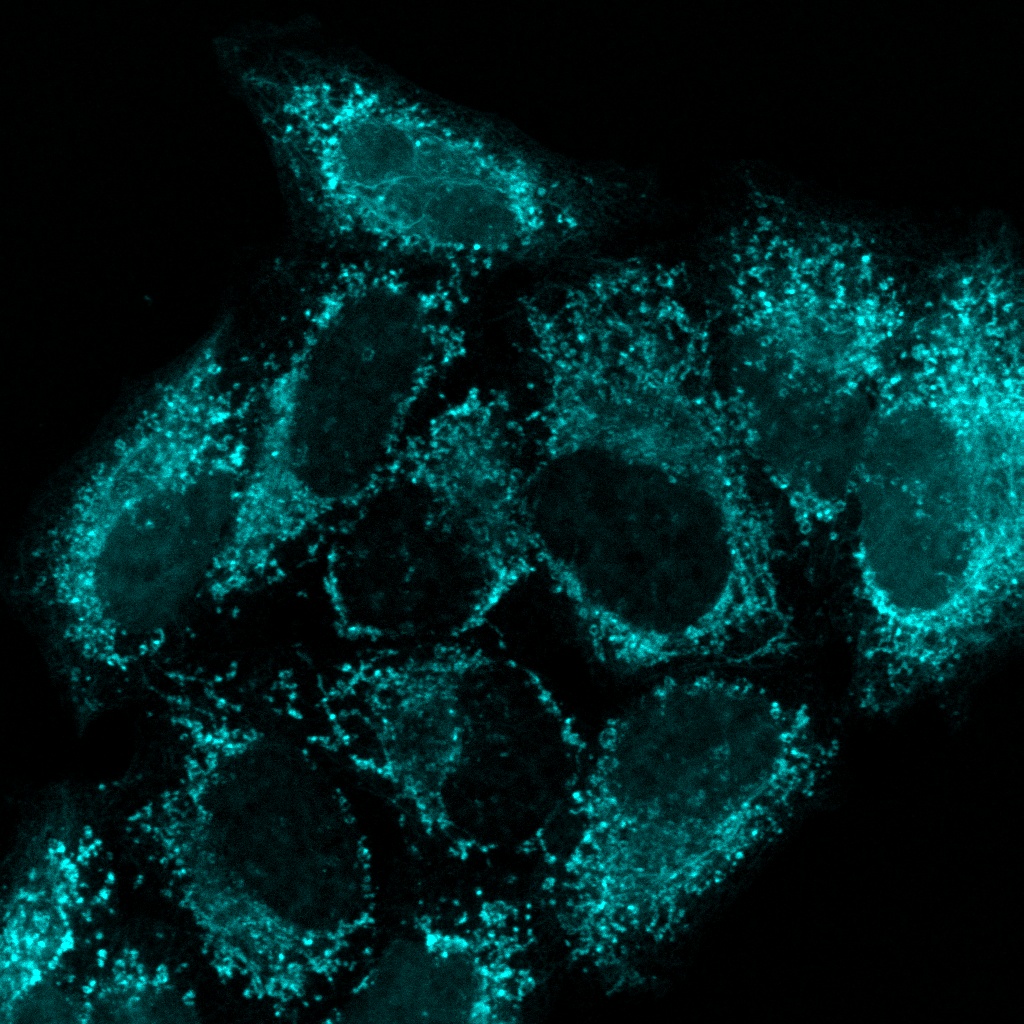
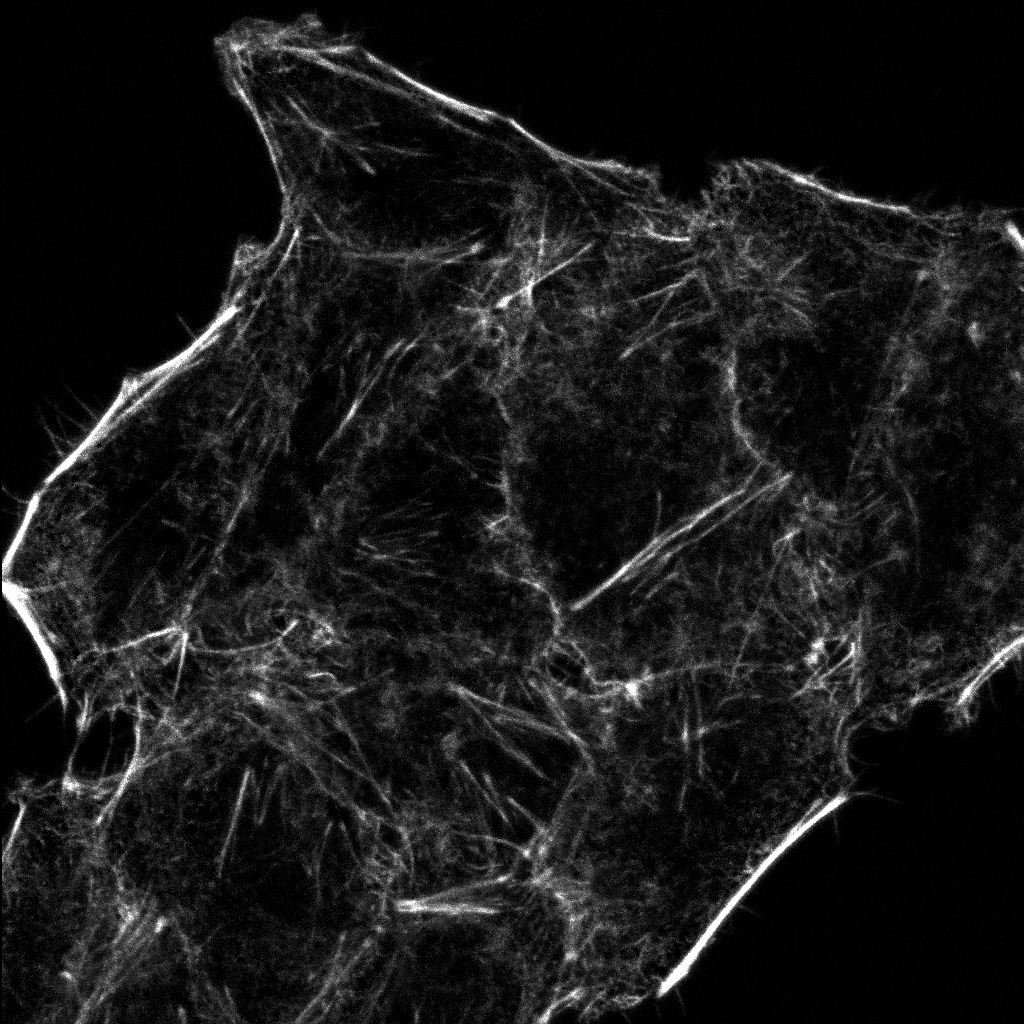
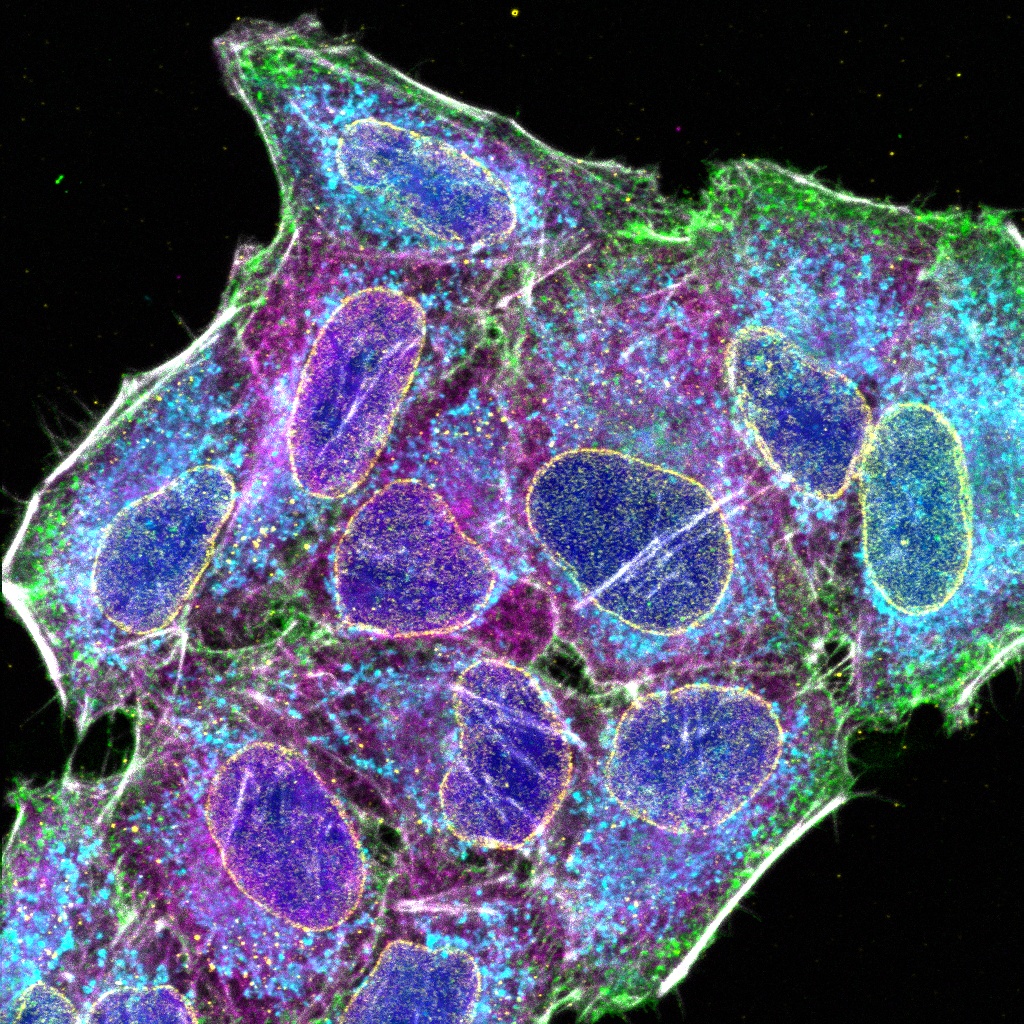
Experience the systems innovations, including:
*As of October 2023. |
Easy-to-Acquire, Quantitative Confocal DataThe FV4000 confocal microscope uses our advanced, silicon-based SilVIR™ detector that makes it easier than ever to acquire precise, reproducible data. SilVIR Next-Generation Detector Technology The SilVIR detector combines two advanced technologies—a silicon photomultiplier (SiPM) and our patented* fast signal processing design.
*Patent number US11237047 |
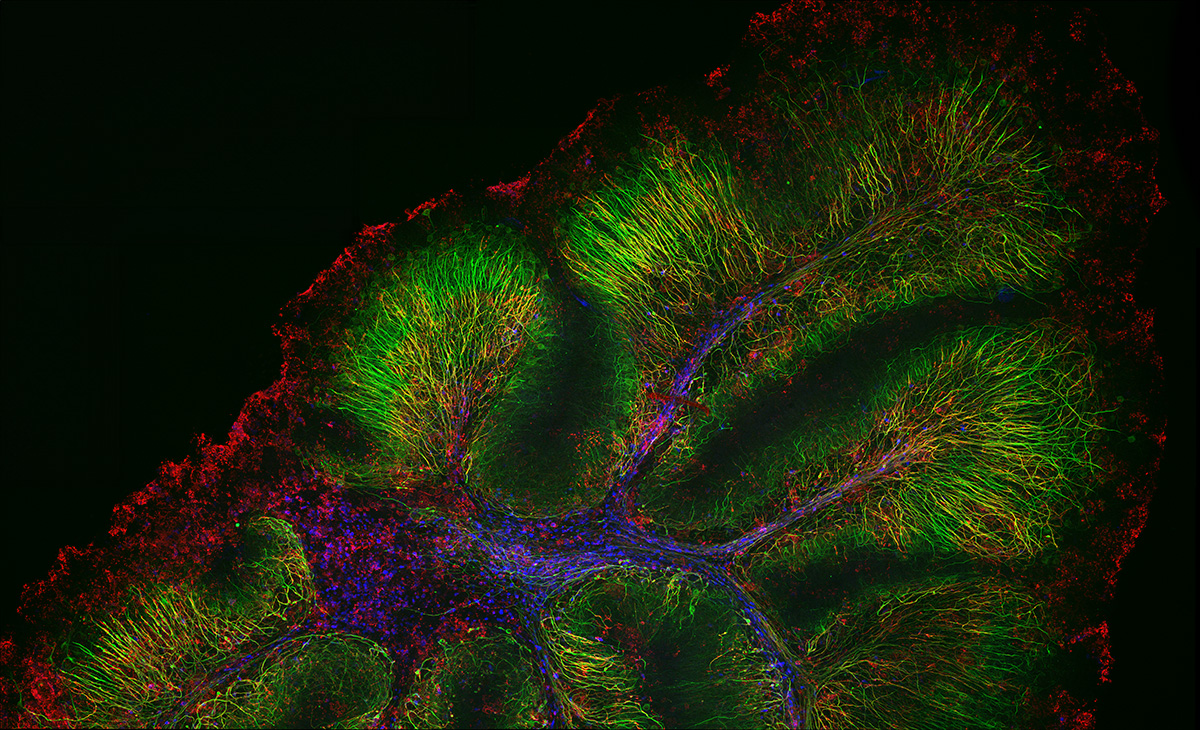
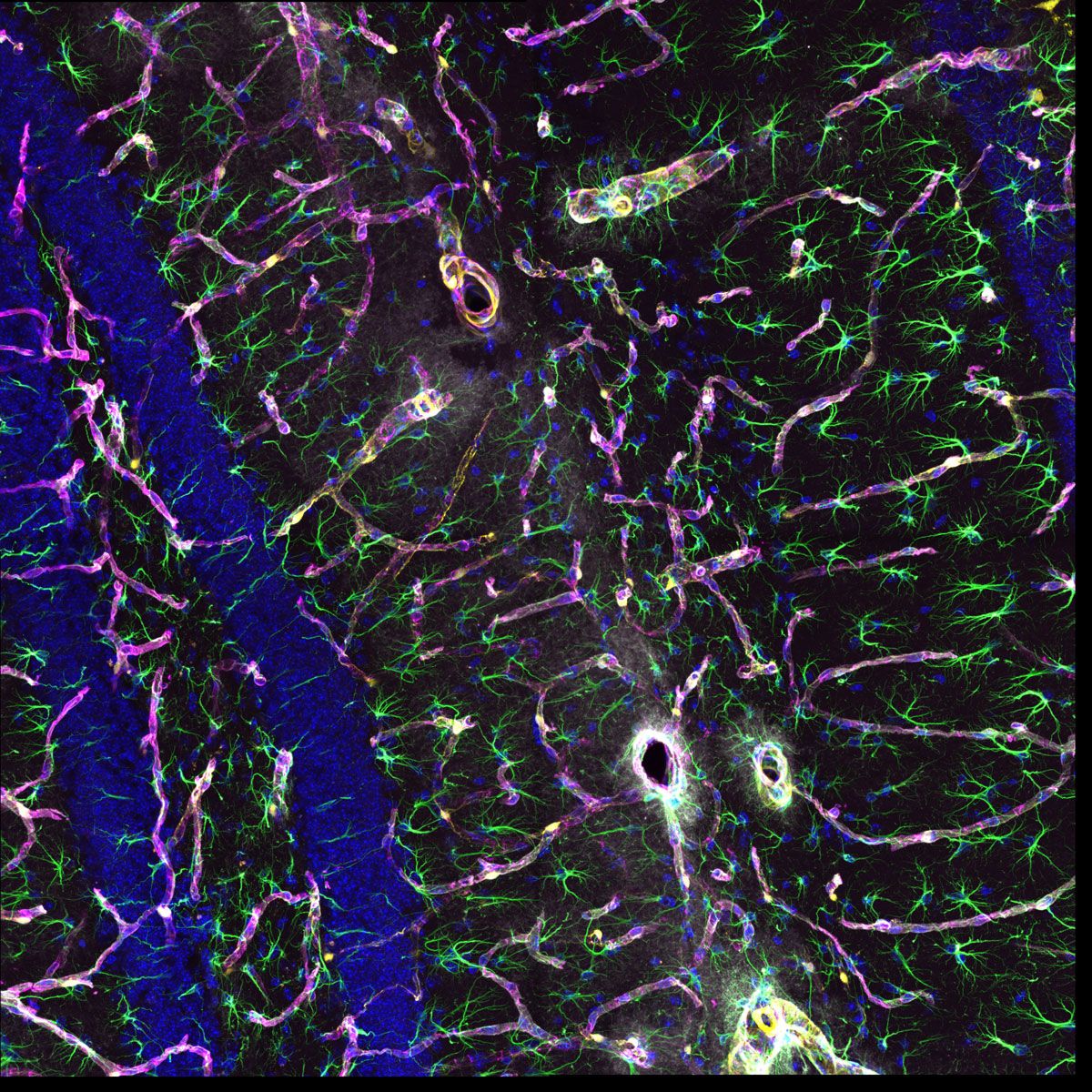
Neurofilament-heavy chain (NFH) in green, myelin basic protein (MBP) in red, glutathione S-transferase pi 1 (GSTpi) in blue. Mouse cerebellum captured with a UPLXAPO40X objective. |
 |
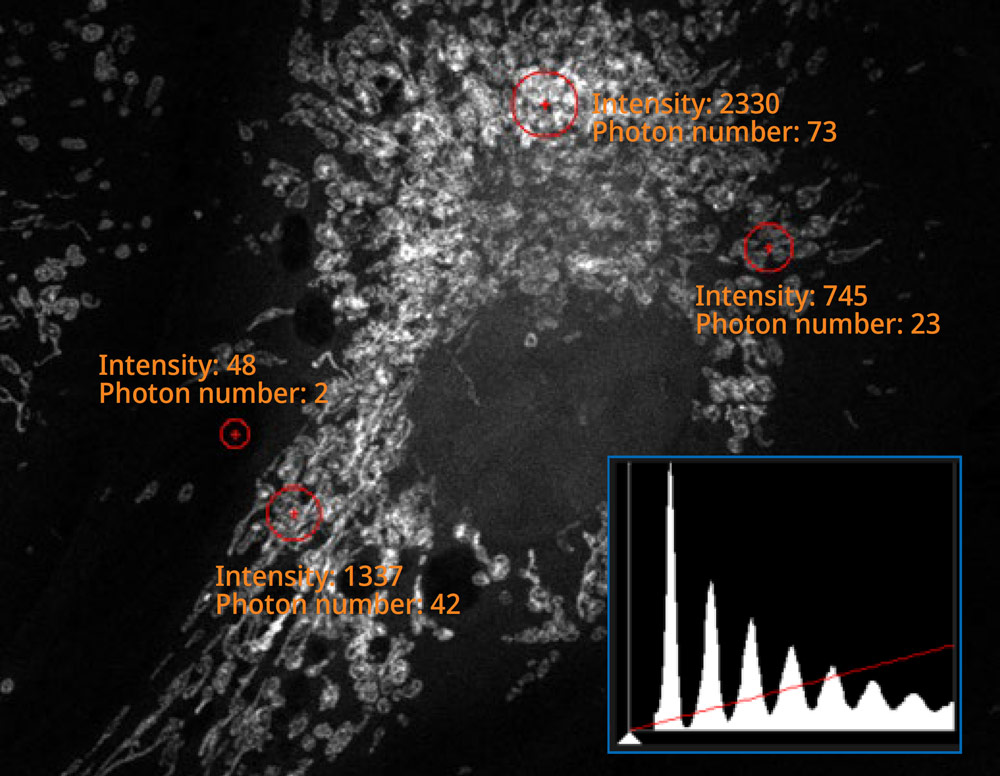 |
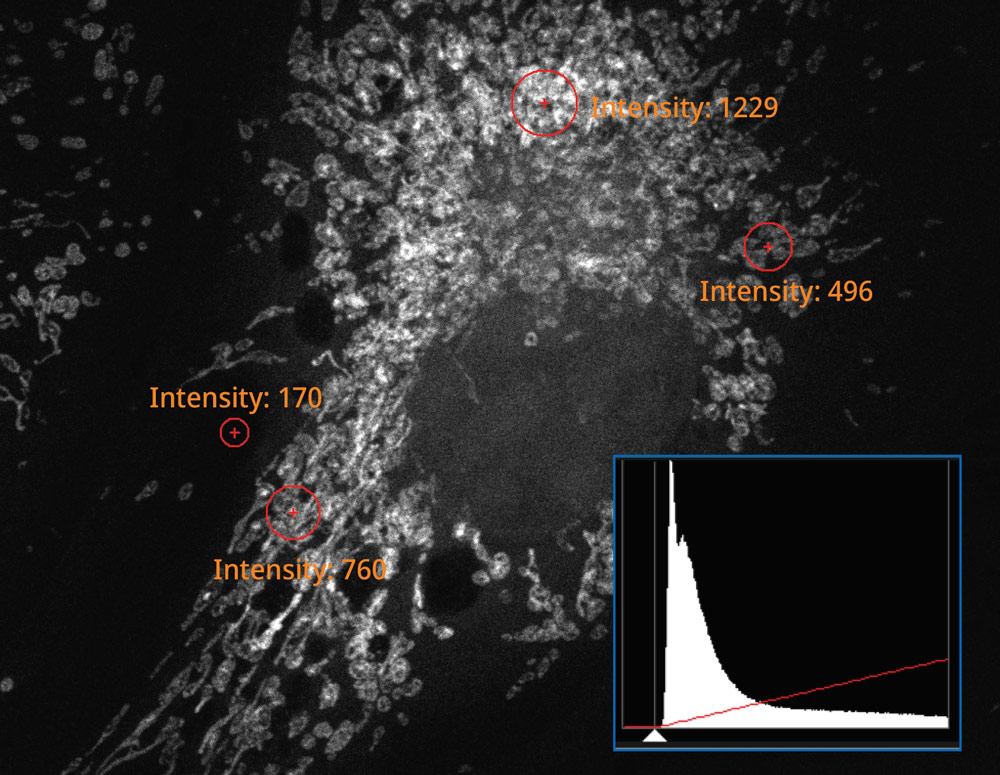
| The histogram on the image captured using the SilVIR detector shows a discrete pattern where the intensity can be converted to the photon number. The detector’s fluorescence intensity can be quantified as the photon number, and the background level is extremely low. |
More Information from Your Confocal ImagesThe system’s updated TruSpectral technology combined with high sensitivity SilVIR detectors enable you to see more by making it possible to multiplex up to six channels simultaneously.
|
Flexible Macro to Micro ImagingThe macro-to-micro workflow enables you to easily observe the target sample from the macro level—whole body or tissue—down to the cell or subcellular level.
|
||||
Gentler High-Speed Time-Lapse Confocal Imaging |
|
|
HeLa cells labeled by MitoView 720. XYZT imaging by 1K resonant scanner for 30 min. |
Time-lapse imaging is easier with smart features:
|
Reproducible Image Data Between Users and SystemsThe SilVIR detector has less sensitivity loss over time compared to previous-generation detector technologies. With our laser power monitor (LPM) and TruFocus™ Z-drift compensator, achieve reproducible images under consistent conditions for better reproducibility. Different users on different days can acquire the same precise images using the same settings. Even the images acquired by different FV4000 microscopes can be compared and discussed using the same photon number intensity scale. |
Microscope Support and Service You Can Count OnWe designed the FV4000 system to be easy to maintain:
We stand behind our products with a commitment to fast service and technical support. We offer various support plans to keep your microscope running at peak performance at a predictable cost as well as remote support options, so you don’t need to wait for an engineer or specialist to visit if you’re having an issue.
|
See Further with NIR-Enabled Confocal MicroscopyThe system’s enhanced technologies provided expanded multiplexing to see more in one image. NIR imaging offers greater multiplexing capabilities by extending the excitation (λ_Ex) and detection (λ_Em) spectral profile of the FV4000 system. This enables additional dyes to be used to help minimize emission signal overlapping.
|
High-Quality Optics for Efficient NIR Fluorescence ImagingThe FV4000 system’s optical elements have a high transmission from 400 nm to 1300 nm, including the galvanometer and resonant scanner, which are coated in silver rather than aluminum. Our award-winning X Line™ objectives are corrected for chromatic aberrations between 400–1000 nm. They also have a higher numerical aperture, excellent flatness, and very high transmittance from UV to NIR, increasing the multiplexing capabilities. For improved colocalization reliability, our specialized A Line™ (PLAPON60XOSC2) oil immersion objective (ne~1.40) significantly minimizes chromatic aberration for strict colocalization analysis. |
 |
|
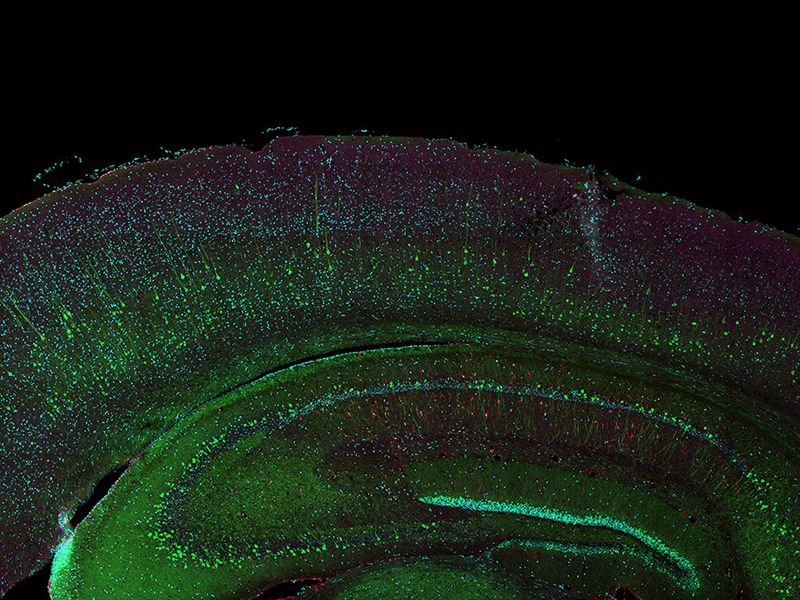
A total of 77 four-channel XYZ positions (11 × 7) were acquired using a 1K resonant scanner within 16 minutes to create the stitched image, which used to require 2 hours using a galvanometer scanner. The coronal section of an H-line mouse brain, cyan; DAPI (cell nuclei), green; YFP (neuron), yellow; Cy3 astrocytes, magenta; AlexaFluor 750 (microtubule). Sample courtesy of: Takako Kogure and Atsushi Miyawaki, Cell Function Dynamics, RIKEN CBS. |
High-Quality Confocal Images at High SpeedA unique combination of advanced technologies delivers high-quality images faster than conventional laser scanning microscope systems.
|
|---|
Simple, Precise Super Resolution ImagingCapture super resolution images using the FV4000 microscope with no dedicated hardware.
|
|
High-Resolution 3D Images in Thick Samples |
|
When imaging thicker samples, the FV4000 microscope enables you to capture high-resolution, 3D images.
|
Precise Dynamics of Live Cells with Less Damage
|
Clear Images at DepthUse our silicone immersion objectives with the FV4000 microscope and achieve clear images of features and structures deep within your sample. Silicone oil has a refractive index close to that of live cells or tissue, greatly reducing the spherical aberration as compared to air, water, or other oils. With less aberration, you can achieve clearer images of your sample at depth. And silicone immersion oil does not dry out at 37 ℃ (98.6 °F), making it effective for long-term time-lapse imaging. |
|